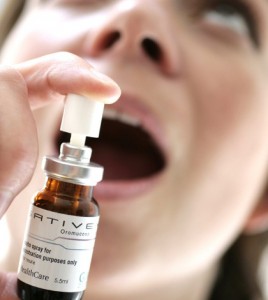
Medical THC comes in many forms: smoked, vaporized, eaten. But it also comes in prescription form, legal in many countries. It’s called Sativex, and it’s the first prescription drug containing marijuana extract. So how does it work?

That is the primary use for Sativex. It can be prescribed to treat that condition in Canada, the United Kingdom, Australia, and Spain, among other countries. It is not yet approved for use in the United States, but that could change.
This form of medical marijuana is poorly understood by many patients. It comes directly from the cannabis plant and is not synthetic, yet it undergoes a different processing regimen. The medication contains all the compounds found in marijuana, and is similar in some ways to CBD oil and hash oil. The chemical makeup is somewhat different, and Sativex is not always effective, but it is close to the original.
Sativex provides standardized dosages
Sativex attempts to answer a common concern of doctors, who often complain there is no way to accurately dose marijuana when the drug is smoked or eaten. The medication allows for standardized dosages and a more regulated effect.
Sativex is made in the United Kingdom, where marijuana is otherwise illegal for any use. GW Pharmaceuticals, which produces the drug, has a special license to grow research cannabis, and Sativex is approved for medical use in the country.
Though it doesn’t typically get users as high as regular marijuana, Sativex carries the same potential side effects. That includes mild intoxication, dizziness, oral discomfort, and fatigue. Tests have confirmed that Sativex has no effect on long-term cognitive function.
Expected to reach U.S. stores in 2016
Though currently illegal in the United States, Sativex could reach drug stores later in 2016. The drug is in the final stage of clinical trials into its usefulness in treating cancer pain. It was fast-tracked by the FDA in 2014, and approval should come this year or next. Sativex is not officially treated as cannabis in the United States, making it easier to clear the medication for use here.
But even with FDA approval, Sativex will likely remain too expensive for many patients. A yearly prescription in New Zealand costs $16,000 in U.S. dollars, enough to buy more than an ounce of high-quality cannabis per week. This means patients often turn to the black market instead, especially because insurers typically won’t cover the bill.
The uses for Sativex could be wider than currently understood. Besides multiple sclerosis and cancer-related pain, it could be used to fight brain cancer, arthritis, and nerve pain. Making it available to more patients, for a more reasonable price, could improve the lives of countless people. For the time being, it isn’t the most effective choice for marijuana medicine, but it offers hope to many suffering patients.













